
It holds values for constants, defines external inputs to the model and calculates algebraic relationships.

The Converter serves a utilitarian role in the software. “Compartments” accumulate whatever flows into them, net of whatever flows out of them, with “Flows” filling and draining accumulations. The program uses Compartments, Flows, Converters, and Connectors as building blocks.
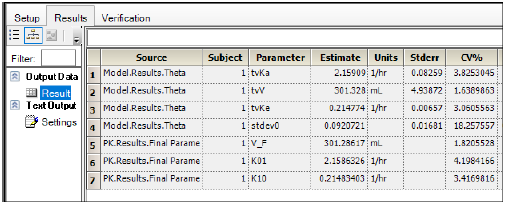
Winnonlin for pk software#
STELLA ® (ISEE Systems Inc., Lebanon, PA, USA) is a simulation software application that enables the study of systems through its graphical representation.

In this model type, tissues are grouped into compartments, depending on their blood flow and drug binding (tissues with similar blood flow and drug tissue binding are grouped in the same compartment). The most used PK model is the compartmentally based, which represents a very simple and useful tool in PK. These models can be divided into three categories, empirically, physiologically, and compartmentally based PK models. Theoretical approaches aim at the development of PK models to predict drug disposition, which includes drugs distribution and elimination after its administration. The study of PK involves both theoretical and experimental approaches. These models not only improve decision making throughout clinical drug development, but also enable the design and optimization of dosing regimens, increasing the chances of the drug to reach its target with the desired concentration and drug plasma concentration to be maintained within the therapeutic window. Pharmacokinetic (PK) models describe the absorption, distribution, metabolism, and the elimination of molecules (drugs, compounds under development, etc.) in an organism, thus providing useful information to foster efficient and informative drug development. New drugs approval takes on average seven to nine years and the cost of introducing a new drug can range from 600 million to 1 billion euros. The process of research and development (R&D) of new drugs is very time consuming and expensive. This work demonstrates that in silico methods and AUC effect are powerful tools to study relationships between tissue drug concentration and the percentage of cell growth inhibition over time. In addition, cell growth inhibition was itraconazole-dose dependent and an increase in effect was predicted if itraconazole administration was continued (24-h dosing interval). Considering the quantification parameter area under the dose-response-time curve (AUC effect) for the combinations effect, itraconazole was the most effective in combination with either reference anticancer drugs. Then, two-compartment PK models were developed based on the previous in vitro studies and on the PK profile reported in the literature for human patients. Combinations of reference drugs for cancer treatment, gemcitabine and 5-fluorouracil (5-FU), and repurposed drugs itraconazole, verapamil or tacrine, were evaluated in vitro. In this work, in silico PK models were developed based on in vitro assays results, with the goal of predicting the in vivo performance of drug combinations in the context of cancer therapy. In complex diseases like cancer, single-agent approaches are often insufficient for an effective treatment, and drug combination therapies can be implemented. Pharmacokinetic (PK) studies improve the design of dosing regimens in preclinical and clinical settings.


 0 kommentar(er)
0 kommentar(er)
